Heat Storage Catalyst Simulation to Enhance Energy Efficiency of Integrated Chemical Reaction
Integrating exothermic and endothermic reactions within a single reactor offers a promising strategy to enhance overall thermal efficiency. However, existing systems often suffer from limited heat transfer performance due to design and heat transfer constraints. To overcome this challenge, we propose a novel catalyst support incorporating latent heat storage, enabling temporary storage of excess heat from exothermic reactions (during the charging/melting phase) and its release to support endothermic reactions (during the discharging/solidification phase) as presented in Figure 1.
Al-Cu-Si microencapsulated phase change material (MEPCM) was tested as a catalyst support in a co-production system combining CO2 methanation (exothermic) and NH3 decomposition (endothermic). This approach improved thermal energy efficiency by approximately 11% and enhanced CO2 conversion during the thermal suppression process. However the quantification of exact heat preserved is remain unknown. Therfore, to analyze the heat exchange behavior between reaction enthalpy, latent heat absorption, and heat loss, a computational fluid dynamics (CFD) simulation was performed using COMSOL Multiphysics 6.2.
The simulation used an axisymmetric cylindrical fixed-bed reactor model incorporating five coupled physics interfaces: laminar flow, transport of diluted species, chemistry, heat transfer in fluid, and Darcy’s law. The reaction mechanism was modeled as a simplified reversible reaction with each kinetic expressions defined in the chemistry module. Reaction enthalpies were implemented as heat sources, while MEPCM phase change behavior was modeled in the porous domain using the thermal properties of the Al-Cu-Si MEPCM composite. Heat loss through the reactor wall was modeled as convective heat transfer. Simulation results were validated against experimental data for both the charging (CO2 methanation) and discharging (NH3 decomposition) phases.
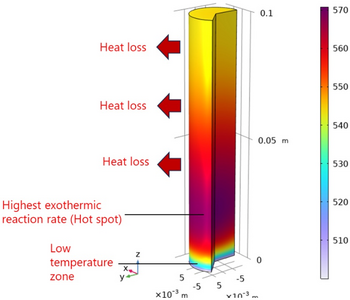
Figure 2 depict the steady-state temperature behavior on the charging step whereas the reactor inlet temperature increased rapidly from 500 °C to a steady state of ~570 °C driven by the strong exothermic reaction. Afterwards, the downstream temperature decreased gradually because of heat loss and reduced reaction rates. Within the transient process itself, once the MEPCM melting point (519 °C) was reached, latent heat absorption began, denoted by thermal suppression along the reactor axis as presented in Figure 3. Temperature readings at multiple points, corresponding to the thermocouple positions used in experiments, show similar trends—featuring a brief thermal suppression near the inlet (due to fast charging) and a prolonged suppression toward the outlet (due to slower charging dynamics).
In accordance with the thermal suppression curve, the transient phase change behavior at Figure 4 shows a rapid phase transformation on the inlet and prolonged on the downstream afterward. Subsequently, the final parameter result of CO2 methanation including temperature profile and phase change state was coupled as the initial value of NH3 decomposition simulation.
Based on the observed characteristic, an inevitable hot spot was observed on the inlet part of the catalyst. On the other hand, large amounts of heat loss led to the uneven temperature distribution and hindered the charging process. Therefore, the future reactor design incorporating MEPCM catalyst will focus on addressing these problems.
This study demonstrates that incorporating MEPCM into the catalyst matrix can significantly improve the energy efficiency of co-production systems. Sensitivity analysis based on validated results provides deeper insight into MEPCM behavior and catalyst design optimization. These findings contribute to advancing thermal energy storage technology and enabling more sustainable and efficient chemical processes.